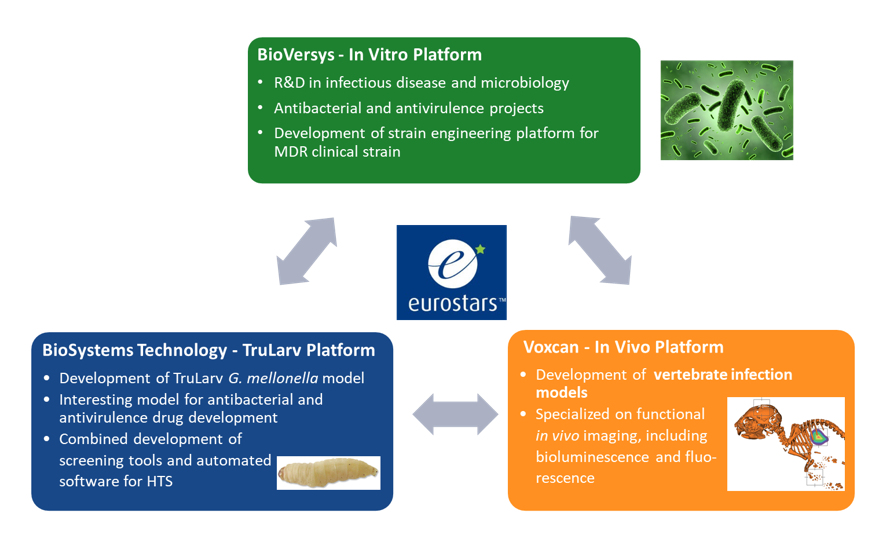
1. The In Vitro Platform, developed by BioVersys
It will provide novel methods to construct genomic alterations in any MDR/XDR clinical isolate of the high priority KAPE pathogens, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, and Escherichia coli. Using these methods, bioluminescent clinical strains will be developed and provided to the project partners for the development of animal models with non-invasive real-time readouts. BioVersys will use the new tools to also provide on-demand bacterial genome engineering to customers.
2. The TruLarv Platform, developed by Biosystems Technology
It will establish translational models with the generated bioluminescent strains that allow tracking of intracellular pathogens by imaging and in vivo proof-of-concept (PoC) in Galleria mellonella larvae. This system can be used to screen clinical isolates for virulence and to test antibacterial compound efficacies and act as a filter before testing on mammals to reduce the number of animals required in screens. BioSystems Technology will develop imaging software for high-throughput antimicrobial compound screening in TruLarv.
3. The In Vivo Platform, developed by Voxcan
It will establish relevant infection models with the same bioluminescent strains that were validated in the TruLarv platform. Generated infection models will allow tracking of the establishment and spread of bacterial infections in the animals in a non-invasive way. The efficacies of test drugs can be determined in such murine models in real time by imaging the load of bioluminescent bacteria in the animals.
